Clinical trials are the bridges between ideas born in theory and their real application in the healthcare industry. These trials are dynamic processes that evaluate the efficacy, and safety of novel medical therapies while ensuring they reach patients on time. Earlier, the collection and assessment of data for these trials was done using a paper-based Case-Report Forms (CRFs), which resulted in a cumbersome and time-consuming process. With the advent of technology, the traditional methods are being replaced with efficient methods like the adoption of Electronic Data Capture (EDC) systems.
What is an Electronic Data Capture (EDC) System in Clinical Trials?
Electronic Data Capture (EDC) system in clinical trials is a software product enabling a streamlined and efficient collection, management and analysis of data electronically. This system ensures the security and authenticity of the data while improving accessibility.
Unlike the traditional paper-based Case-Report Forms (CRFs), an EDC system is a comprehensive tool that effectively eliminates the concerns related to:
- Data integrity due to human error
- The enormous pressure associated with paper-based documentation
- The lengthy duration of clinical trials
Offering these advantages, EDC has emerged as a crucial technology that can be widely used in pharmaceutical companies, healthcare institutions and clinical research organizations (CROs).
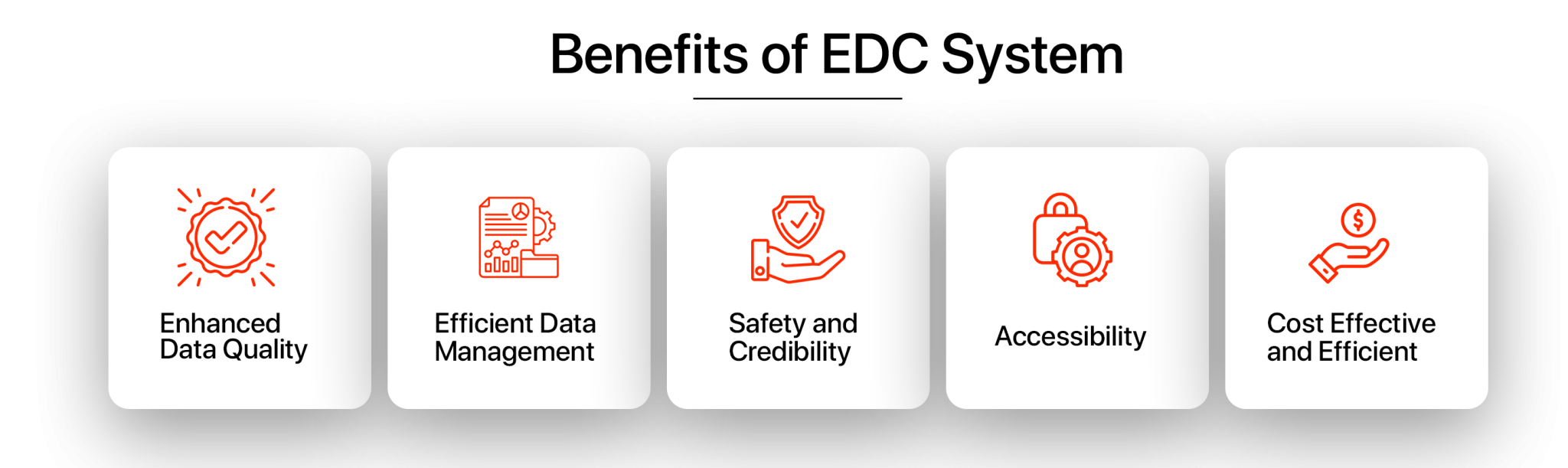
Benefits Of Using Electronic Data Capture (EDC) System In Clinical Trials
Medical device and pharmaceutical companies have started incorporating Electronic Data Capture (EDC) Systems for the optimization and maintenance of the data acquired during clinical trials. The EDC system has fundamentally changed the way clinical trials are performed, making it important to understand the benefits that it brings along.
The five benefits of using EDC systems for capturing data electronically in clinical trials have been mentioned below.
Enhanced Data Quality
Electronic Data Capture (EDC) systems significantly enhance data quality. It minimizes errors due to manual data entry and transcription through in-built validation and edit checks. Real-time monitoring of the data allows the user to detect discrepancies ensuring their accuracy and credibility. Inconsistencies are automatically flagged and this helps in guaranteeing that the data is correct and reliable.
Efficient Data Management
An EDC system provides a centralized platform that offers real-time access to authorized individuals which simplifies the process of data collection, input, storage, retrieval and organization. This optimizes the data workflow and consistency, promotes collaboration, avoids data silos and is time efficient.
Safety and Credibility
The data security is greatly prioritized in an EDC system. The data is stored in a secure cloud storage which avoids data breaches, loss of data and fragmentation. Only authorized individuals can access the data and carry modifications, ensuring transparency and accountability at every stage.
Accessibility
The EDC serves as a storehouse for unified data which only users with authorization can access. This enables researchers, regulatory authorities and associated institutions to study and analyze the data in real-time, and in turn, enhance communication, collaborative insights and decision-making. Accessibility fosters synergism among the associated individuals and it creates an enhanced workflow with positive outcomes.
Cost Effective and Efficient
By ensuring enhanced quality of the data through streamlined operations and remote monitoring, an EDC system minimizes the time required for data preparation and cleaning. Elimination of paper forms and related manual tasks not only saves time but reduces the cost as well. Moreover, real-time access to the data avoids the laborious task of visiting the clinical trial sites and cuts down on travel expenses.
Evolution of Clinical Trials
In the journey of progress, evolution is a constant companion. To adapt to the evolving healthcare landscape, it is necessary to update ourselves by embracing efficient technology. Electronic Data Capture is a key player in the advancement of medical science as it offers a multitude of advantages like enhanced data quality, efficient data management, security and accessibility. It favours a collaborative atmosphere enabling transparency, authenticity and well-informed decision-making. Further, the cost and time-effective nature of these systems also contribute to enhancing the efficiency of the clinical trials.
Clinion offers the best-in-class, certified – EDC solution which enables automation and acceleration of clinical trials. The latest version of the Clinion EDC Platform is backed by AI and offers management of clinical trials across all phases with full transparency. The user-friendly interface supports the efficient collection, cleanup, reporting and accessibility of your clinical trial data. In short, our product is an all-in-one solution designed to optimize your research endeavors.