Formulated on the advancements in the field of drug development, the pharmaceutical industry has been progressing at a rapid pace. But despite significant investments in the development of modern therapies and treatments, the overall efficiency of pharmaceutical R&D efforts have been declining steadily. The increasing timelines and costs associated with clinical trials has been widely recognized as a key contributor to the trend.
Factors Paving the Way for Decentralization of Clinical Trials
The pressure of the current epidemic has brought into greater focus the need for revolutionizing the functioning of the pharmaceutical sector. Vaccine development has set the benchmark for the development of effective medicines at a hitherto unseen, unprecedented speed. The lessons being learnt during the pandemic offer rich insights into the future course of the clinical research industry including insulation from further major disruptions. Decentralized Clinical Trials (DCT) are predicted to offer an implicit defense against major disruptions.
DCTs are defined as studies “executed through telemedicine and mobile or local healthcare providers, using processes and technologies that are different from the traditional clinical trial model.” However this does not imply performing clinical trials without the supervision of healthcare professionals, rather its about looking at areas where technology and other novel solutions can enable sponsors and CROs to take a more practical approach towards designing clinical trials that can provide an alternative to the current site-anchored, inflexible system which often results in a high patient drop out rate.
Hence, keeping in mind the challenges of the traditional trial concepts as well as the ones faced by the new decentralized practices, a resolution has been sought in Hybrid trials that have since emerged as the new paradigm for conducting clinical trials.
Benefits and Challenges of Decentralized Clinical Trials
Patient availability and participation have turned out to be persistent problems in the conventional model. The goal of modernizing clinical trials is to make it easier for subjects to participate in trials by reducing the need for them to visit specific sites.
Benefits
- Increased patient engagement
- Decreased study costs and timelines
- Stramlined data collection
- Easier recruitment efforts
- Better access to diverse patient populations
Challenges
- Patient safety
- Privacy factors- Technology glitches or failures
- IP delivery, management and processing
- High volumes of patient data
- Data from various monitoring sources and wearables.
Statistics have revealed that 70% of patients reside more than two hours away from research sites. In 50% of clinical trials, participants find it difficult to stay enrolled due to poor health and the majority of trials fail to retain enough patients till the end. Remote trials technology helps in reaching out to a diverse population, lessening their physical efforts at participation.
Although decentralization addresses a majority of these difficulties faced by site based trials, there are certain barriers that cannot be overlooked.
While it’s important to be aware of all the challenges, the benefits decentralized trials offer for patient recruitment and retention outweigh the potential risks which – with efficient partners, vendors, and planning– can be sufficiently mitigated.
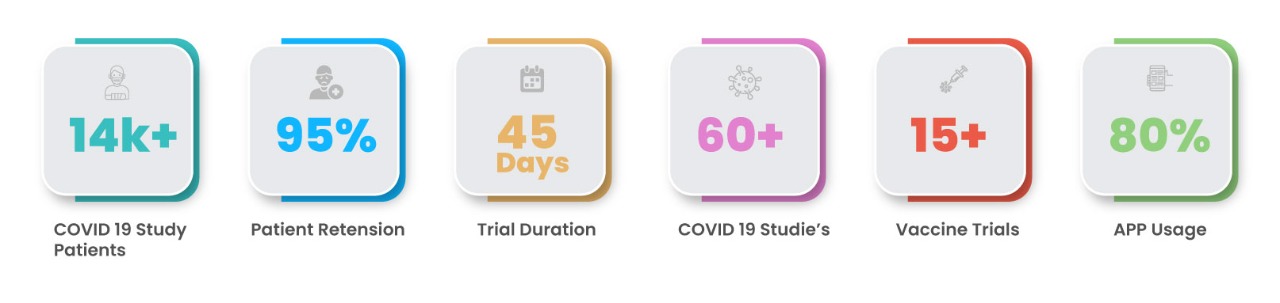
Case Study Revelations
These numbers are highly encouraging as it exhibits the willingness of patients’ to participate using remote trial technology.
Decentralised Clinical Trials – The Future of Clinical Research
There is no doubt about the fact that virtual and hybrid trials have gained momentum since they were first introduced. The industry is working relentlessly to identify tools, infrastructure and collaborations required to enable trials that are more patient-centric than site-centric. The desired partners will be CROs that have diverse potential in drug development and beyond, have the technical prowess to generate insights with their data which will enable them conduct more efficient clinical trials.