Integrated Medical Imaging
Clinion DICOM
Image Repository
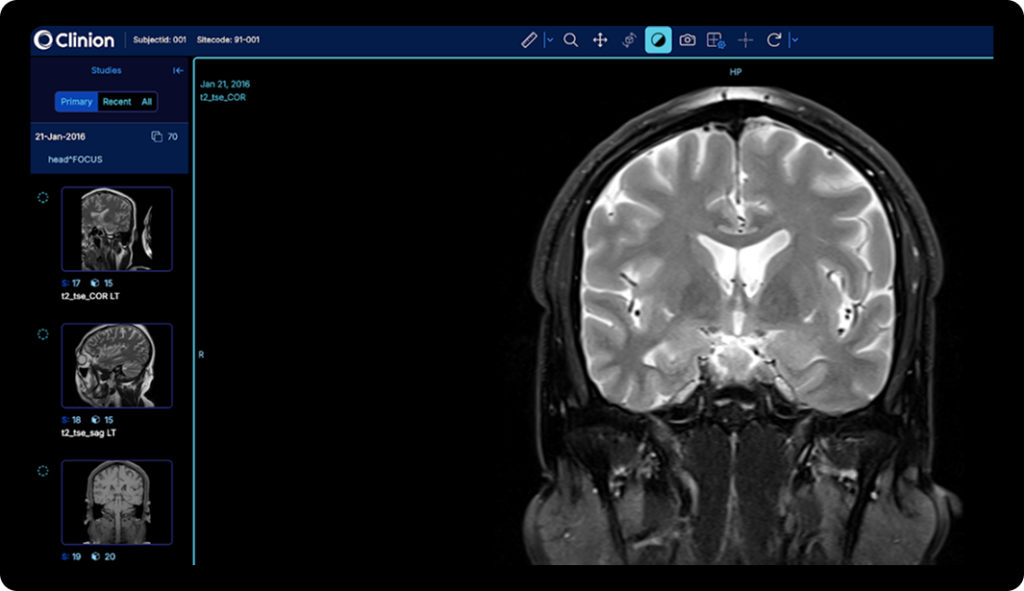
Results
Transform Medical Image Management with Measurable Results
Clinion’s DICOM Image Repository transforms how clinical teams handle medical imaging, enabling them to upload, de-identify, and access trial images with complete automation, all while ensuring compliance, eliminating data silos, and maintaining perfect, audit-ready traceability.
PHI Redaction Time
De-identify images automatically in under 30 seconds with AI-powered detection and removal.
Imaging Modalities
Support for CT, MRI, PET, Ultrasound, X-ray, Mammography, Pathology, and more.
Global Access
Browser-based viewing and export for sponsors and sites across all time zones.
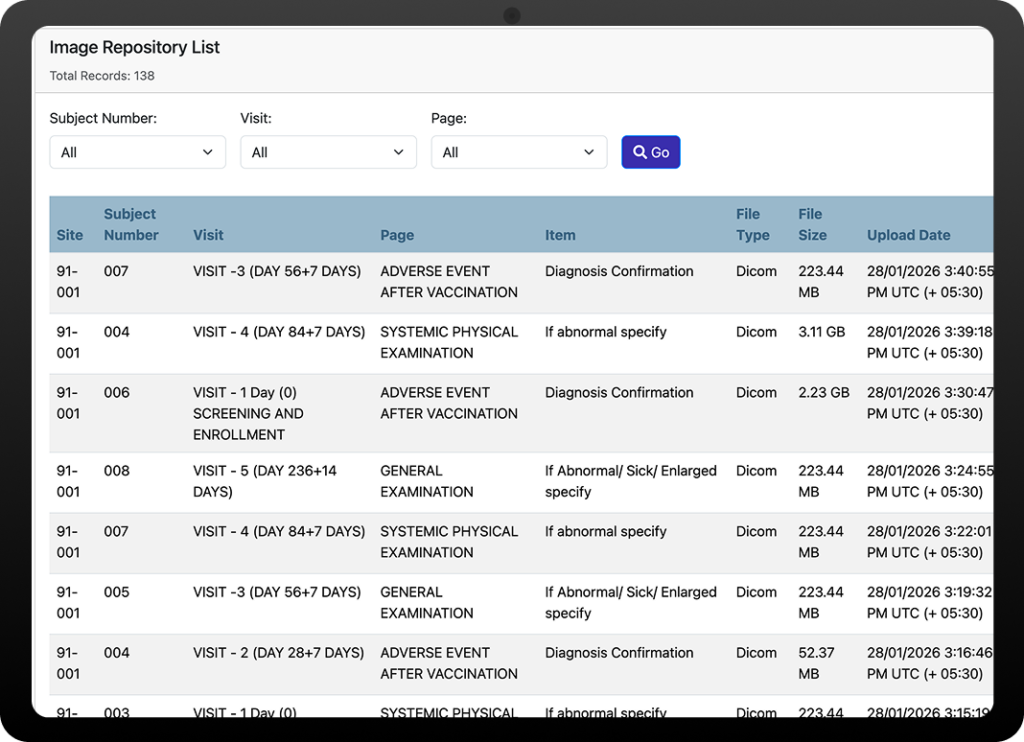
Streamline Your
Imaging Workflow in Five Steps
Upload Your Images
Upload medical images in any format - DICOM, JPEG, PNG, or PDF. Browser-based submission works from anywhere, with pre-upload validation to catch errors early.
AI Redacts PHI Automatically
Our AI scans every image to detect and remove patient identifiers including patient name, DOB, gender, and site details in seconds.
Store Securely in the Cloud
Images are encrypted and stored on AWS HealthImaging, organized by subject, visit, and page with direct links to corresponding CRF data in Clinion EDC.
Visualize with Built-in Tools
View and analyze images using our integrated DICOM viewer - MPR, 3D rendering, fusion workflows, without needing external PACS or software installation.
Export and Report On-Demand
Download individual images or batch export for sponsors, sites, and regulators. Comprehensive audit reports ensure you're always inspection-ready.
Clinion Difference
Fully integrated into your EDC
Unlike standalone imaging platforms, Image Repository lives inside Clinion EDC. Every image is linked to subjects, visits, and CRF pages, eliminating data silos and manual reconciliation.
Single login for all trial data
Real-time sync with EDC subject records
One-click navigation from image to CRF page
Easy access to images from eCRF pages
Image viewing capability within EDC
Multi-Image compare capability within EDC
Easy report access within EDC
Benefits
Why Teams Choose Integrated Imaging
Faster Study Timelines
Automated PHI redaction and direct EDC linking eliminate manual steps, reducing image processing time from hours to seconds and accelerating overall study delivery.
Lower Total Cost of Ownership
No separate platform to procure, validate, or maintain. No additional IT infrastructure. No extra vendor contracts. Imaging is simply part of your EDC.
Reduced Compliance Risk
AI-powered redaction ensures consistent PHI removal across every image. Unified audit trails and built-in validation keep you inspection-ready without extra effort.
Simplified Site Experience
Sites upload images in the same system they use for data entry – one login, one workflow, no context-switching. Less training, fewer errors, faster adoption.
Complete Data Continuity
Every image is linked to its subject, visit, and CRF page from the moment of upload. No reconciliation, no data silos, no gaps in your trial record.
Global, 24/7 Access
Browser-based viewing means sponsors, sites, and monitors can access de-identified images from anywhere, anytime – no software installation required.
AI-Powered Redaction
De-identify Images Automatically In Seconds
Clinion’s AI scans every uploaded image to detect patient identifiers – patient name, dates of birth, gender, site details, and embedded metadata. Sensitive data is redacted before storage.
For studies requiring additional oversight, enable human QC review, to verify all appropriate de-identification is complete.
- AI detects PHI across all image formats (DICOM, JPEG, PNG, PDF)
- Optional human QC review for high-risk studies
- Pre-upload screening catches errors before submission
- Processing completes in seconds, not hours
- End-to-end encryption with AWS Health Imaging
- HIPAA compliant
Interactive Demo
Clinion DICOM Image Repository
Take a guided tour through our end-to-end imaging workflow from each key perspective. See how Site Coordinators upload DICOM, JPEG, or PDF files with instant validation, while AI automatically detects and redacts patient identifiers. Then, step into the Medical Monitor’s role to visualize images with built-in DICOM tools, and explore how Sponsors access compliant, de-identified data linked directly to CRF pages – all without a standalone imaging platform.
Imaging Capabilities
Support for Every Imaging Modality
From CT and MRI to digital pathology – the full spectrum of medical imaging.

CT

MRI

PET

Ultrasound

DX/CR

Mammography

Angiography

RT

Pathology

Other
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion Image Repository platform queries
Clinion Image Repository is an AI-powered medical imaging module built directly into Clinion EDC. It allows clinical teams to upload, de-identify, store, view, and export trial images within a single platform. Unlike standalone imaging systems, every image is automatically linked to subjects, visits, and CRF pages, eliminating data silos and manual reconciliation.
When an image is uploaded, Clinion's AI automatically scans it to detect patient identifiers including name, date of birth, gender, site details, and embedded metadata. PHI is redacted in under 30 seconds before storage. For high-risk studies requiring additional oversight, you can enable optional human QC review to verify de-identification is complete.
Image Repository supports DICOM, JPEG, PNG, and PDF uploads. It covers 10+ imaging modalities including CT, MRI, PET, Ultrasound, X-ray, Mammography, Angiography, Pathology, RT, and more, covering the full spectrum of medical imaging used in clinical trials.
Image Repository lives inside Clinion EDC, not as a separate system. Every uploaded image is automatically linked to the corresponding subject, visit, and CRF page. Users get one-click navigation from image to CRF, real-time sync with subject records, multi-image comparison, and a unified audit trail, all with a single login.
Yes. Image Repository is fully compliant with FDA 21 CFR Part 11, HIPAA, GDPR, ISO 27001:2013, and EU Annex 11. Images are encrypted end-to-end and stored on AWS HealthImaging. Comprehensive audit trails ensure you're always inspection-ready.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Ready to simplify your imaging workflow?
See how Image Repository can transform your clinical trials.
Compliance
Fully Compliant with Global Standards



