Insights / Blog / RTSM
A Comprehensive Guide to Clinical Trial Supply Management and Operations
- Abriti Rai
- February 18, 2026

On this Page
- Summary
- Why Clinical Trial Supply Management Matters
- Why Traditional Supply Management Systems Fall Short
- How Modern Systems Transform Trial Supply Operations
- Role of Technology and AI in Trial Supply Management
- Benefits of a Modern Trial Supply Management
- Best Practices for Effective Clinical Supply Management
- Advanced Considerations for Scalable Clinical Supply Management
- The Evolving Future of Clinical Trial Supply Management
- Summary
- Why Clinical Trial Supply Management Matters
- Why Traditional Supply Management Systems Fall Short
- How Modern Systems Transform Trial Supply Operations
- Role of Technology and AI in Trial Supply Management
- Benefits of a Modern Trial Supply Management
- Best Practices for Effective Clinical Supply Management
- Advanced Considerations for Scalable Clinical Supply Management
- The Evolving Future of Clinical Trial Supply Management
Summary
Clinical trial supply management is the backbone of successful clinical research, ensuring that investigational products reach study sites on time, in optimal condition, and in compliance with global regulations. Effective clinical trial supply chain management ensures trials run smoothly, safeguards patient safety, and preserves the integrity of clinical data.
Modern clinical trials, including decentralized designs, global studies, and personalized therapies, demand a deep understanding of clinical supply operations. Leveraging advanced technology and AI-driven tools is essential to maintain operational efficiency, regulatory compliance, and patient-centric outcomes.
Why Clinical Trial Supply Management Matters
The stakes in clinical trials are high. A delay in delivering investigational products or a lapse in temperature-controlled logistics can lead to:
- Trial interruptions
- Compromised patient safety
- Increased operational costs
- Delayed drug approvals
Example: Delays in delivering temperature-sensitive biologics caused a clinical trial to pause for two weeks, showing how crucial proper clinical supply chain management and monitoring are for maintaining timelines and patient safety.
A well-managed clinical supply chain ensures:
- Timely delivery of investigational products
- Consistent quality and regulatory compliance
- Efficient clinical supply inventory management
- Enhanced patient experience with uninterrupted treatment
Impact Table: Poor vs. Strong Supply Management
Aspect | Poor Supply Management | Strong Supply Management |
Trial Timelines | Delays, missed milestones | On-time dosing and completion |
Patient Safety | Risk of compromised product | Ensures integrity and efficacy |
Costs | Increased wastage & operational costs | Reduced waste and efficient resource use |
Regulatory Compliance | Audit risks, non-compliance | Documentation & adherence across regions |
Why Traditional Supply Management Systems Fall Short
Many clinical trials still rely on manual processes and legacy supply management systems. While these systems may have worked for simpler trials in the past, they often struggle with the complexity and scale of modern clinical studies.
Key Limitations of Traditional Supply Systems
Limitation | Impact on Clinical Supply Operations |
Manual Processes & Fragmented Systems | Delayed reporting and poor coordination across sites and vendors |
Static Inventory Tracking | Inefficient stock management, risk of overstocking or shortages |
Limited Cold Chain Monitoring | Temperature-sensitive products may degrade, affecting safety and efficacy |
Slow Response to Mid-Study Changes | Difficulty adapting to protocol amendments, patient enrollment changes, or unexpected disruptions |
Compliance & Documentation Gaps | Higher audit risks, potential trial delays, and regulatory non-compliance |
Traditional supply management systems often lack the flexibility, real-time visibility, and intelligence needed to manage complex clinical trials, making clinical supply operations challenging and error-prone.
How Modern Systems Transform Trial Supply Operations
While traditional supply management systems struggle with visibility, responsiveness, and compliance, modern Trial Supply Management Systems are designed to handle the complexity of today’s clinical trials. By integrating all aspects of clinical supply operations, a modern system manages the entire lifecycle of clinical material supply, from planning and procurement to post-trial returns and reconciliation.
Key Components of a Modern Clinical Trial Supply Management System
To understand how these systems streamline operations, we can break down the key components of a modern supply management systems:
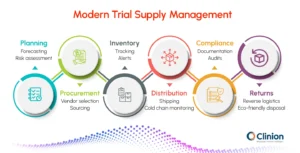
Planning & Forecasting
Estimate supply needs based on patient enrollment, dosing schedules, and study duration. Use predictive analytics to minimize shortages and overstock.
Procurement
Source investigational products and ancillary materials while ensuring compliance. Efficiently manage vendor relationships and contracts.
Inventory Management
Maintain optimal stock levels across multiple sites. Track lot numbers, expiry dates, and storage conditions.
Distribution & Logistics
Ensure timely delivery using temperature-controlled transport. Manage customs and cross-border shipping efficiently.
Regulatory Compliance
Adhere to GMP, GCP, GDP, and local regulations. Automate documentation to simplify audits and inspections.
Returns & Reconciliation
Track unused supplies and dispose of them safely. Incorporate eco-friendly solutions for sustainability.
Modern CTSMS transforms clinical supply operations from reactive and fragmented processes into streamlined, data-driven, and patient-focused systems. These systems ensure the right supplies reach the right place at the right time.
Role of Technology and AI in Trial Supply Management
Modern clinical trial supply management systems increasingly rely on technology and artificial intelligence (AI) to handle the growing complexity of clinical trials. These tools help teams improve efficiency, reduce errors, and ensure patient safety across the supply chain.
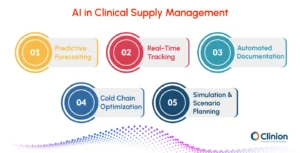
Key Use Cases of Technology and AI
Predictive Forecasting
AI algorithms analyze historical trial data, patient enrollment trends, and site performance to provide more accurate supply predictions. This reduces the risk of overstocking or stockouts and helps sponsors allocate resources efficiently.
Real-Time Tracking'
IoT-enabled sensors monitor the location, temperature, and condition of investigational products throughout the supply chain. This ensures visibility at every stage, allowing teams to respond quickly to potential issues.
Automated Documentation
Natural Language Processing (NLP) and digital platforms generate regulatory documents automatically. This reduces manual effort, minimizes human error, and ensures faster compliance with audits and inspections.
Cold Chain Optimization
Advanced sensors detect temperature deviations in real time, helping maintain the integrity of temperature-sensitive biologics, vaccines, and other investigational products. Proactive monitoring reduces spoilage and ensures patient safety.
Simulation & Scenario Planning
Technology can model potential disruptions such as shipment delays, site closures, or protocol changes. By simulating different scenarios, teams can optimize resource allocation and build contingency plans before problems arise.
AI vs Traditional Supply Management
Feature | Traditional Systems | AI-Enabled CTSMS |
Forecasting | Manual estimates, spreadsheets | Predictive and adaptive models |
Inventory Management | Static, periodic checks | Optimized in real time with alerts |
Compliance | Paper-based documentation | Automated regulatory reporting and audit trails |
Cold Chain Monitoring | Reactive checks | Proactive real-time monitoring and alerts |
Disruption Management | Slow response | Simulation, scenario planning, and risk modeling |
Benefits of a Modern Trial Supply Management
Adopting a modern Clinical Trial Supply Management System delivers tangible, measurable value across clinical supply operations. By replacing manual processes with connected, data-driven workflows, these systems help sponsors and CROs run trials more efficiently, compliantly, and sustainably.
Cost Efficiency:
Optimized clinical supply inventory management reduces wastage, overproduction, and unnecessary procurement, helping organizations achieve 15–20% reduction in overall supply costs.
Faster Trials:
Streamlined logistics, real-time visibility, and proactive supply planning prevent dosing delays and resupply issues, contributing to trial timelines that are 1–2 weeks faster on average.
Regulatory Confidence:
Automated documentation, audit trails, and standardized workflows support consistent compliance across sites, enabling continuous audit readiness and reducing regulatory risk.
Transparency:
Real-time dashboards provide end-to-end visibility into inventory levels, temperature conditions, and shipment status, ensuring complete operational oversight across all trial sites.
Patient-Centricity:
Reliable supply availability and enhanced cold chain monitoring help maintain up to 99% temperature compliance for sensitive investigational products, ensuring uninterrupted and safe patient dosing.
Sustainability:
Smarter forecasting, optimized transportation, and improved packaging strategies reduce excess material use, leading to up to 30% reduction in packaging waste across the supply chain.
Best Practices for Effective Clinical Supply Management
Adopting structured best practices helps clinical teams reduce risk, control costs, and maintain uninterrupted trial operations across sites.
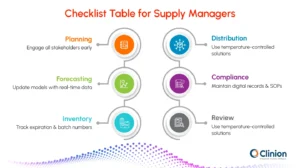
Early Planning & Cross-Functional Collaboration:
Engage sponsors, CROs, vendors, and sites early in the study lifecycle to align expectations, define responsibilities, and anticipate supply risks before trial activation. This minimizes last-minute changes and avoidable delays.
Integrated Supply Chain Strategy:
Design supply operations in line with protocol requirements, enrollment projections, and geographic complexity. A unified strategy ensures supply decisions support study objectives while accounting for risks, contingencies, and scalability.
Accurate Forecasting:
Use predictive models and historical trial data to estimate demand based on enrollment rates, dosing schedules, and site performance. Accurate forecasting reduces overstocking, prevents stockouts, and supports cost-efficient inventory planning.
Vendor Management:
Work with qualified and reliable suppliers for packaging, labeling, logistics, and distribution. Conduct periodic audits and performance reviews to ensure vendors meet quality, compliance, and delivery standards.
Temperature Control & Monitoring:
Implement validated packaging solutions and continuous temperature monitoring to protect sensitive investigational products. Real-time alerts help teams respond quickly to excursions and maintain product integrity.
Performance Metrics & Visibility:
Track key metrics such as On-Time In-Full (OTIF) delivery, inventory wastage, shipment delays, and temperature deviations. These insights enable proactive decision-making and early issue resolution.
Continuous Improvement:
Regularly review KPIs, deviations, and operational outcomes to refine processes. Ongoing optimization helps teams adapt to changing trial conditions and improve supply chain resilience over time.
Advanced Considerations for Scalable Clinical Supply Management
As clinical trials expand in scale, geography, and complexity, supply strategies must move beyond foundational best practices. These advanced considerations help organizations future-proof their clinical supply operations while maintaining efficiency, compliance, and patient focus.
Sustainability
Adopt eco-friendly packaging, optimize shipment routes, and reduce excess inventory to minimize waste and lower the environmental footprint of clinical trials without compromising supply reliability.
Patient-Centric Supply Models
Support decentralized and hybrid trials through direct-to-patient delivery models. Simplified kit designs and flexible resupply approaches improve patient adherence and reduce dependency on site-based logistics.
Globalization and Regionalization
Effectively navigate varying regulatory requirements across regions while leveraging regional depots or distribution hubs. This approach minimizes cross-border delays, improves responsiveness, and enhances supply continuity.
Advanced Performance Metrics
Move beyond basic tracking by continuously monitoring OTIF delivery, temperature excursions, wastage levels, and stock utilization rates. These metrics enable proactive decision-making and sustained optimization across global studies.
The Evolving Future of Clinical Trial Supply Management
Clinical trial supply management is evolving in response to how trials are being designed and executed today. AI-driven forecasting is improving how teams plan supply quantities by accounting for enrollment variability, dosing patterns, and site performance, reducing both shortages and excess inventory.
As decentralized and hybrid trials expand, supply models are shifting toward direct-to-patient delivery and more flexible distribution strategies that support home-based dosing and remote participation. At the same time, the rise of personalized therapies is driving smaller, more precise clinical material supply approaches, where accuracy and timing matter more than scale. These changes are also pushing organizations to adopt automation, smart tracking, and sustainable logistics practices that improve operational reliability while reducing waste and environmental impact.
Looking ahead, modern clinical supply operations will be defined by their ability to adapt quickly without adding complexity. Teams that invest in intelligent planning, real-time visibility, and patient-centric distribution models will be better equipped to manage mid-study changes, global regulatory expectations, and increasingly fragile or high-value therapies. The focus is shifting from simply moving supplies efficiently to ensuring continuity, predictability, and resilience across the entire trial lifecycle, setting a stronger foundation for faster studies, better patient experiences, and more reliable outcomes in the trials of tomorrow.
Clinion RTSM
Designed to manage both randomization and supply within a single platform, Clinion RTSM supports studies of varying complexity while maintaining accuracy, compliance, and operational control. On the supply side, it provides end-to-end visibility across depots, countries, and clinical sites, enabling teams to manage inventory, re-supply, expiry, temperature excursions, and returns from one system. Automated supply checks and intelligent re-supply logic help prevent stockouts, protect product integrity, and ensure every transaction is captured within a GxP-compliant audit trail, all while working seamlessly with Clinion EDC to reduce manual effort and system silos across global trials.
Also Read: RTSM in Clinical Trials: A Guide to Randomization and Trial Supply Management

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
Clinical trial supply management ensures investigational products reach trial sites on time, in the right quantity, and under proper conditions. Effective management minimizes delays, reduces waste, and safeguards patient safety throughout the study.
A modern Clinical Trial Supply Management System integrates planning, inventory, distribution, and compliance in one platform. It reduces errors, streamlines logistics, and provides real-time visibility across sites and depots.
AI helps predict supply needs, optimize inventory, and identify potential disruptions before they occur. It enables smarter forecasting, reduces stockouts, and supports proactive decision-making.
Modern systems automate documentation, track temperature excursions, and maintain audit-ready records. This ensures adherence to GMP, GCP, and GDP standards across all trial sites and countries.
Many investigational products, especially biologics, require strict temperature ranges. Continuous monitoring and cold chain management protect product integrity and ensure patient safety.
Decentralized trials require direct-to-patient shipments and smaller kit sizes, increasing logistical complexity. Modern CTSMS and AI-powered tracking enable seamless distribution while maintaining compliance.
Key metrics include On-Time In-Full (OTIF) delivery, inventory levels, wastage, and temperature excursion rates. Monitoring these KPIs allows continuous improvement and risk mitigation.
Clinion RTSM unifies patient randomization and supply workflows, providing end-to-end visibility across sites and depots. Automated re-supply, expiry management, and integration with EDC reduce errors and manual effort.
Yes. Optimizing transportation routes, using eco-friendly packaging, and reducing excess inventory lowers environmental impact while improving cost efficiency.
Emerging trends include AI-driven forecasting, decentralized trial logistics, personalized medicine kits, and warehouse automation. These innovations enhance efficiency, compliance, and patient-centricity.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


