Insights / Blog / AI in Clinical Trials
AI in Clinical Trials: Use Cases, Benefits & Future Trends
- Abriti Rai
- January 28, 2026

On this Page
- Summary
- Introduction: Clinical Research at an Inflection Point
- Why Traditional Clinical Trial Models Are No Longer Sufficient
- The Role of AI in Clinical Research
- Regulatory Considerations for AI in Clinical Trials
- The Future of AI-Driven Clinical Trials
- Conclusion
- External References
- Summary
- Introduction: Clinical Research at an Inflection Point
- Why Traditional Clinical Trial Models Are No Longer Sufficient
- The Role of AI in Clinical Research
- Regulatory Considerations for AI in Clinical Trials
- The Future of AI-Driven Clinical Trials
- Conclusion
- External References
Summary
AI in clinical trials is addressing the challenges of escalating data complexity and accelerated timelines. AI in clinical trials is being used to handle increasing complexity, larger volumes of data, and shorter development timelines. It enhances not only operational efficiency but also the underlying approach to study design and management, facilitating faster and more reliable insights for strategic decisions.
Introduction: Clinical Research at an Inflection Point
Clinical trials remain the definitive mechanism for validating the safety, efficacy, and real-world applicability of new therapies and medical devices. Yet despite decades of methodological refinement, the operational realities of clinical research have become increasingly strained. Trial complexity has risen sharply, timelines continue to extend, and costs have escalated to levels that challenge both innovation velocity and patient access.
One of the clearest indicators of this shift is data scale. While eligibility criteria for clinical trial participants have remained largely consistent over the past decade, the volume of data generated in Phase III trials has tripled, reaching approximately 3.6 million data points per study, compared to levels observed ten years ago. This expansion is driven by the proliferation of electronic health records (EHRs), wearables, imaging, genomic datasets, decentralized trial technologies, and real-world data sources. Traditional operational models were not designed to manage, analyze, or act upon data at this magnitude or velocity.
At the same time, persistent structural inefficiencies remain unresolved:
- Nearly 80% of trials fail to meet enrollment timelines
- Protocol amendments remain common, costly, and disruptive
- Site performance variability continues to undermine predictability
- Manual oversight dominates areas that demand continuous, real-time intelligence
Against this backdrop, AI in clinical trials is no longer experimental or aspirational. It is becoming an operational necessity.
Industry benchmarks increasingly demonstrate measurable impact:
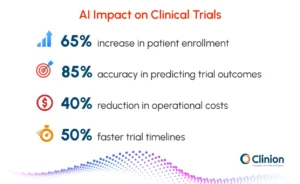
- AI-powered patient recruitment tools improve enrollment rates by up to 65%
- Predictive analytics models achieve approximately 85% accuracy in forecasting trial outcomes and site performance risks
- AI integration accelerates trial timelines by 30-50% while reducing operational costs by up to 40%
These gains are not the result of isolated tools, but of a broader shift toward clinical trial automation, where artificial intelligence and machine learning are embedded across the trial lifecycle.
This article examines how AI and machine learning in clinical trials are reshaping trial design, execution, monitoring, and evidence generation, while also addressing the regulatory, ethical, and operational constraints that govern their adoption.
Why Traditional Clinical Trial Models Are No Longer Sufficient
Clinical trials today operate in an environment characterized by:
- Multi-country, multi-site execution
- Increasingly narrow patient populations
- High protocol complexity
- Continuous data inflow from heterogeneous sources
Yet operational decision-making often relies on periodic reviews, static reports, and retrospective analysis. This disconnect between data availability and actionable insight is a fundamental limitation of legacy models.
Structural Inefficiencies Across the Trial Lifecycle
Key friction points persist across studies:
| Trial Phase | Persistent Challenges |
| Trial Design | Overly restrictive eligibility criteria, limited real-world relevance |
| Site Selection | Reliance on historical assumptions rather than predictive performance |
| Recruitment | Manual screening, low patient awareness, and high dropout rates |
| Trial Conduct | Reactive monitoring, delayed issue detection |
| Data Management | Manual cleaning, delayed locks, reconciliation bottlenecks |
| Reporting | Resource-intensive CSR development, long close-out cycles |
These inefficiencies compound over time, increasing both direct costs and opportunity costs.
The Role of AI in Clinical Research
Early adoption of automation in clinical trials focused on digitization - electronic data capture, electronic trial master files, and basic workflow automation. While valuable, these systems largely replicated manual processes in digital form.
In contrast, AI in clinical research introduces capabilities that are fundamentally different:
- Pattern recognition across high-dimensional datasets
- Probabilistic forecasting rather than rule-based triggers
- Continuous learning from historical and real-time data
- Decision support at scale
AI does not merely accelerate tasks; it alters how decisions are made.
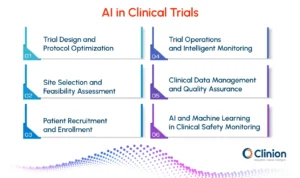
AI-Driven Trial Design and Protocol Optimization
Re-evaluating Eligibility Criteria Using Data
Eligibility criteria play a critical role in patient safety and scientific validity, yet they are often inherited from prior protocols without empirical reassessment. This contributes to unnecessarily narrow recruitment pools and reduced generalizability.
AI and machine learning models trained on historical trial and real-world datasets can:
- Identify criteria with minimal impact on safety or endpoints
- Quantify trade-offs between inclusivity and statistical power
- Simulate enrollment and outcome scenarios under modified criteria
Outcome: Broader, more representative cohorts without compromising trial integrity.
Adaptive and Predictive Trial Design
AI enables pre-trial simulation and ongoing optimization through:
- Predictive enrollment modeling
- Endpoint sensitivity analysis
- Scenario-based protocol feasibility testing
These capabilities reduce late-stage amendments and improve operational realism before first-patient-in.
AI in Site Selection and Feasibility Assessment
From Retrospective Metrics to Predictive Performance
Traditional site selection relies heavily on historical enrollment numbers and subjective feasibility questionnaires. AI-driven models expand this analysis by incorporating:
- Past enrollment velocity and screen failure rates
- Data entry timeliness and query resolution patterns
- Protocol deviation history
- Local patient population characteristics
- Competing trial density
Benefits of AI-Enabled Site Selection
- Reduced site over-activation
- Higher probability of on-time enrollment
- Early identification of underperforming sites
- More balanced geographic and demographic representation
This represents a shift from reactive site management to predictive site intelligence.
AI-Powered Patient Recruitment and Enrollment
Patient recruitment remains the most significant driver of delays and cost overruns. Despite advances in outreach and awareness, manual screening processes struggle to keep pace with data availability.
AI Improves Recruitment Outcomes by leveraging:
- Natural language processing to parse unstructured EHR data
- Machine learning models to match patients against eligibility criteria
- Conversational agents to support pre-screening and engagement
Measured Impact:
- Up to 65% improvement in enrollment rates
- Faster screening cycles
- Reduced screen failures
- Improved patient retention through personalized engagement
AI enables precision matching at scale, transforming recruitment from a bottleneck into a controllable process.
AI-Enabled Trial Operations and Intelligent Monitoring
Intelligent Workflow Automation
AI-enabled workflow automation introduces a fundamental shift. Rather than executing predefined rules, AI systems continuously analyze operational data, identify emerging risk patterns, and dynamically adjust priorities across trial activities. Workflows evolve from static sequences into adaptive, intelligence-driven systems.
Real-time enrollment intelligence
AI models track enrollment velocity at site, country, and cohort levels, continuously benchmarking performance against predictive forecasts. Early deviations enable intervention before timelines are compromised.
Predictive protocol adherence monitoring
By learning from historical deviations, visit compliance trends, and site behavior, AI identifies where protocol non-compliance is likely to occur, before deviations materialize.
Proactive data quality risk detection
Machine learning algorithms surface subtle inconsistencies, anomalous value distributions, and missing data patterns in near real time, reducing downstream rework and late-stage delays.
Risk-based task prioritization
Monitoring activities, queries, and follow-ups are dynamically ranked based on impact and likelihood, ensuring operational effort is aligned with risk rather than uniform schedules.
Multi-Agent AI Systems for Coordinated Trial Intelligence
As clinical trials generate increasingly complex and interdependent signals, single-model AI architectures face practical limitations. Multi-agent AI systems address this challenge by deploying specialized AI agents that operate autonomously within defined domains while coordinating through shared objectives and constraints.
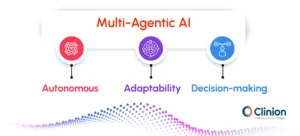
In clinical trial operations, this architecture enables parallel intelligence across enrollment, protocol execution, data quality, safety, and site performance, without centralizing all decision logic into a single model.
Within a multi-agent framework:
- Protocol intelligence agents interpret protocol logic in real time, monitor deviation risk, assess amendment impact, and align downstream activities with protocol intent
- Enrollment agents continuously assess recruitment velocity, screen failure patterns, and cohort balance
- Site performance agents monitor operational behavior, deviation trends, and data timeliness
- Data quality agents evaluate consistency, anomaly patterns, and reconciliation risks across systems
- Safety agents track evolving risk signals across clinical, wearable, and patient-reported data
Each agent operates independently on its respective data streams while contributing structured signals to a shared decision layer. When conflicting signals emerge, such as accelerated enrollment alongside rising protocol deviations, these tensions are surfaced explicitly, enabling informed human oversight rather than opaque automation.
Risk-Based Monitoring at Scale
Risk-based monitoring has long been recognized as a best practice, yet its adoption has been limited by manual review cycles and fragmented data. AI in clinical trials enables RBM to function as a continuous, system-level capability.
AI-driven monitoring frameworks analyze high-frequency data streams to detect anomalies across multiple dimensions:
Safety signal evolution, integrating adverse events, laboratory trends, and patient-reported outcomes.
Data integrity risks are identified through cross-form, cross-site, and cross-system consistency analysis.
Site performance deviations, including query resolution behavior, visit compliance, and data entry patterns.
Patient adherence risks, inferred from visit attendance, ePRO completion, and device data continuity.
By synthesizing these signals in real time, AI shifts monitoring from retrospective review to proactive intervention. Oversight becomes targeted rather than blanket, reducing unnecessary on-site activity while strengthening quality control and inspection readiness.
AI in Clinical Data Management and Quality Assurance
Managing Data at Modern Trial Scale
With millions of data points generated per study, manual data review is neither scalable nor timely. AI in clinical data management introduces system-level intelligence into workflows, enabling continuous oversight rather than episodic review. Machine learning models analyze data as it is generated, identifying quality risks before they propagate across downstream processes.
Key capabilities include:
Automated discrepancy detection
AI models surface inconsistencies across forms, visits, and systems, distinguishing meaningful anomalies from expected variability.
Predictive query generation
Rather than reacting to obvious errors, algorithms anticipate likely data issues based on historical patterns, triggering queries earlier in the data lifecycle.
Pattern-based anomaly identification
Subtle deviations in data distributions, entry behavior, or timing are detected through comparative and longitudinal analysis.
Intelligent cross-system reconciliation
AI supports alignment across EDC, ePRO, laboratory, and external data sources, reducing manual reconciliation effort and late-stage bottlenecks.
Impact on Data Timelines and Trial Readiness
- Faster availability of clean data for interim analyses
- Earlier and more predictable database locks
- Reduced rework during study close-out
- Improved inspection readiness through continuous traceability
Critically, AI does not replace data management expertise. It augments it- filtering noise, prioritizing attention, and enabling data managers to focus on high-impact decisions rather than exhaustive manual review.
AI and Machine Learning in Clinical Safety Monitoring
Safety oversight in modern trials extends beyond periodic safety reviews and static listings. Today’s studies generate safety-relevant signals from multiple sources, including-
- Clinical assessments
- Wearable devices
- Imaging
- Patient-reported outcomes
AI and machine learning models enable continuous safety surveillance by synthesizing these heterogeneous data streams into unified analytical frameworks. Rather than evaluating signals in isolation, models assess patterns across modalities and time.
Core capabilities include:
Early detection of adverse event patterns
Machine learning models identify emerging trends that may not be apparent through traditional rule-based thresholds.
Patient-specific risk trajectory modeling
Longitudinal analysis enables the identification of individuals or subgroups at elevated risk, supporting earlier clinical intervention.
Prioritized medical review workflows
Safety signals are ranked by potential impact and urgency, allowing medical reviewers to focus on the most clinically relevant insights.
Regulatory Considerations for AI in Clinical Trials
Regulatory authorities, including the FDA and EMA, increasingly acknowledge the role of AI in modernizing clinical research. However, acceptance is conditional. Rather than evaluating AI as a standalone innovation, regulators assess it through the lens of risk, accountability, and scientific validity.
Alignment with AI Governance Standards (ISO/IEC 42001)
As AI adoption matures, organizations are increasingly aligning their clinical AI systems with formal governance standards. ISO/IEC 42001, the international standard for AI management systems, provides a structured framework for governing AI across its lifecycle.
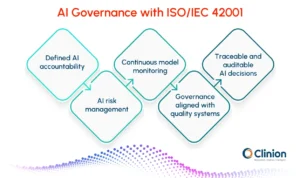
Relevance of ISO/IEC 42001 to Clinical Research
In the context of clinical trials, alignment with ISO/IEC 42001 enables organizations to operationalize Responsible AI principles consistently and measurably by supporting:
- Defined accountability structures
Clear assignment of roles and responsibilities for AI development, validation, deployment, and oversight, ensuring that decision authority remains traceable and auditable. - AI-specific risk management
Systematic identification and mitigation of risks related to model bias, performance drift, unintended outcomes, and patient safety impact, beyond traditional software validation. - Lifecycle performance monitoring
Continuous evaluation of AI models post-deployment to ensure ongoing accuracy, reliability, and fitness for intended use as trial conditions and data distributions evolve. - Governance integration with quality systems
Alignment of AI oversight with existing clinical quality management frameworks, including GxP, risk-based monitoring, and inspection readiness processes. - Traceability and documentation
Structured documentation of data sources, model training, validation decisions, and change management, supporting both internal audits and regulatory inspections.
While not a regulatory requirement, such standards signal organizational maturity and preparedness, particularly as regulators place greater emphasis on governance, traceability, and accountability.
Responsible AI in Clinical Research
Beyond formal regulatory pathways, there is increasing emphasis on Responsible AI as a foundational requirement for clinical adoption. Responsible AI in clinical trials is anchored in a defined set of principles that ensure patient safety, regulatory compliance, and operational trust.
Accountability: Clear ownership of AI-enabled decisions, with defined roles for oversight, escalation, and intervention across the trial lifecycle.
Transparency: Explainable models and documented decision logic that allow regulators, sponsors, and investigators to understand how outputs are generated.
Privacy and Security: Robust data protection mechanisms, consent management, and secure data handling aligned with global privacy regulations.
Reliability and Safety: Consistent model performance across populations and geographies, supported by continuous validation and monitoring for performance drift.
Fairness: Active identification and mitigation of bias to ensure equitable treatment of diverse patient populations and representative trial outcomes.
These principles are increasingly scrutinized during inspections, audits, and sponsor due diligence processes.
Area | Regulatory Focus |
Data Quality | Representativeness, completeness, and auditability |
Model Transparency | Explainability, documentation, and decision traceability |
Governance | Human-in-the-loop oversight and escalation controls |
Validation | Continuous performance monitoring and revalidation |
Privacy | Data protection, consent management, and security safeguards |
The Future of AI-Driven Clinical Trials
As AI, automation, and real-world data mature together, clinical research is moving away from static, milestone-driven execution toward systems capable of continuous sensing and informed adaptation.
Future trial ecosystems will increasingly be characterized by:
- Persistent intelligence layers that operate across design, execution, and close-out rather than within isolated functions
- Decision environments that update continuously, replacing episodic reviews with context-aware insight streams
- Protocols that anticipate variability, allowing controlled flexibility without compromising scientific validity
- Evidence generation models that extend beyond trial boundaries, improving relevance to real-world clinical practice
This represents a structural shift in clinical research, from managing processes to managing intelligence.
Organizational Readiness as a Competitive Divider
As AI becomes embedded within trial operations, differentiation will depend less on access to technology and more on organizational readiness. Sponsors and CROs that operationalize AI through defined governance, quality alignment, and cross-functional ownership will be better positioned to scale their impact responsibly.
Key strategic differentiators will include:
- The ability to govern AI systems as regulated assets, not experimental tools
- Integration of AI oversight within existing clinical quality and risk frameworks
- Workforce models that combine domain expertise with AI-augmented decision support
- Cultural acceptance of data-driven adaptation as a core operational norm
In this context, AI maturity becomes an organizational capability rather than a technical feature.
Conclusion
AI in clinical trials is no longer evaluated by novelty or potential, but by how effectively it reshapes decision-making under real operational constraints. As trial complexity, data volume, and regulatory expectations continue to rise, the limitations of traditional execution models become increasingly apparent.
The long-term value of AI and automation in clinical trials will be realized not through isolated efficiency gains but through disciplined integration, where intelligent systems are governed, explainable, and embedded within the clinical research lifecycle. The future of clinical development will be defined by those who treat AI not as an accelerator of existing processes, but as a foundation for more adaptive, accountable, and resilient clinical research.
Clinion AI: Redefining Clinical Trials
Clinion’s AI-powered eClinical platform delivers a comprehensive and fully integrated suite of solutions, encompassing EDC, RTSM, CTMS, eConsent, ePRO, eSource, eProtocol Automation, CSR Automation, and eTMF. By combining AI and intelligent automation across these tools, the platform streamlines every aspect of a clinical trial, from study design and patient recruitment to data management, monitoring, and reporting, ensuring operational continuity, faster timelines, higher data quality, and a more efficient, end-to-end trial experience.
External References
Artificial intelligence and clinical trials: a framework for effective adoption
AI and innovation in clinical trials | npj Digital Medicine
Artificial Intelligence and the future of clinical trials - PMC
AI in Clinical Research: Opportunities, Limitations, and What Comes Next
AI to become as foundational as the internet in clinical trials, experts say

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
AI automates repetitive tasks, predicts enrollment trends, monitors protocol adherence, and detects data quality issues early. This reduces delays, lowers operational costs, and allows teams to focus on high-impact decisions.
AI continuously analyzes trial data to suggest operational adjustments, such as optimizing patient cohorts, reallocating resources to high-performing sites, and reducing the need for late-stage protocol amendments.
Multi-agent AI uses specialized agents for enrollment, site performance, data quality, safety, and protocol management. Each agent operates independently but shares insights with a central layer, enabling scalable, coordinated decision-making.
ISO/IEC 42001 is the international standard for AI management systems. It provides a framework for accountability, risk management, performance monitoring, and integration of AI governance into quality management systems. Aligning with it signals maturity and regulatory readiness.
Responsible AI ensures accountability, transparency, privacy, reliability, and fairness in AI-driven trials. It requires explainable models, clear oversight roles, secure data handling, consistent performance, and bias mitigation.
AI continuously monitors millions of data points, identifies anomalies, generates predictive queries, and reconciles data across EDC, ePRO, lab, and external systems. This ensures faster, more reliable data for analysis and submission readiness.
Yes. AI identifies high-risk sites, predicts potential adherence issues, and monitors deviations in real time. Multi-agent systems can even track protocol logic continuously to prevent errors before they occur.
AI synthesizes safety signals, site performance, patient adherence, and data integrity risks to prioritize monitoring efforts. This reduces unnecessary on-site visits while maintaining high-quality oversight.
No. AI augments human decision-making by filtering noise, highlighting risks, and providing predictive insights. Trial teams remain essential for interpreting findings, making clinical judgments, and ensuring ethical oversight.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


